Professor Niklaus Schaefer started working in nuclear medicine in 2008, long before this relatively new field of medicine became fashionable. Since then, he and his team at the CHUV have treated over a thousand patients with radioligand therapy, an incredibly complex and delicate form of treatment. Niklaus explained to us how radioisotopes can be harnessed to develop personalised medicine in oncology and why it’s a promising therapy.
What is radioligand therapy?
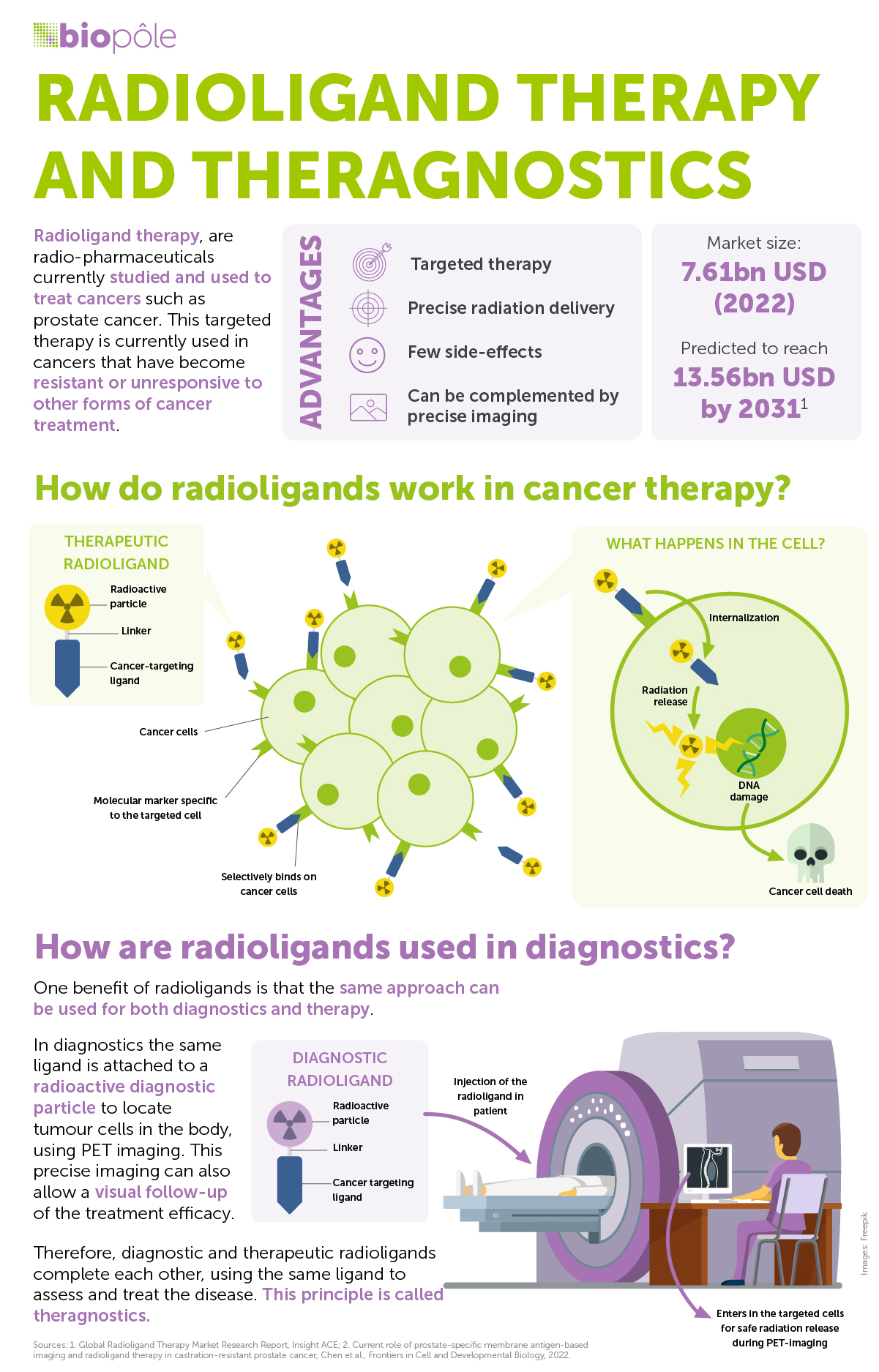
Radioligand therapy (RLT) is an advanced nuclear medicine procedure used to treat specific types of cancers. The two key components in RLT are targeting ligands and radioisotopes. It works by combining the ligand, a molecule that binds to identified cancer cells in the body, with the radioisotope, a radioactive moiety that destroys the target tissue. Because the ligand selectively attaches to targeted cells, the internal irradiation from the therapeutic isotope can be delivered precisely to specific tumour tissue. Consequently, it can eliminate cancer cells while sparing the surrounding healthy tissue, making the treatment highly localised and effective.
RLT is used in diagnosis and therapy. How different is its use in each?
RLT is a form of antitumour therapy that uses internal, local irradiation to destroy cancer cells. RLT is guided by diagnostic imaging: the same ligand is attached to a diagnostic isotope to delineate and quantify the target tumours within the body. Therefore, diagnostic and therapeutic radioligands complete each other, using the same vector to measure and treat the disease. This principle is called theranostics or, more specifically, radiotheranostics.
When is RLT used?
RLT can be used for any cancer where either the tumour cells or cells of the tumour microenvironment feature a specific structure (i.e. a receptor) that can be targeted by its complementary radioligand. For prostate cancer, the prostate-specific membrane antigen (PSMA) receptor can be specifically targeted by the PSMA inhibitor radioligand. Alongside other researchers, we are currently trying to expand this concept to other types of cancer, such as glioblastoma (an aggressive brain tumour), soft tissue sarcoma and breast cancer.
Because RLT is a relatively new field, right now it’s mostly used for patients with advanced cancers, where more common forms of therapy – such as classical chemotherapy – have failed. That said, as the field progresses and an increasing number of clinical trials show the value of RLT, it is being used earlier and earlier.