Radioligand therapy, or RLT, is an innovative cancer treatment that is generating considerable interest in the medical community. In this article, Rüdiger Schenk discusses the challenges and rewards of this targeted nuclear therapy.
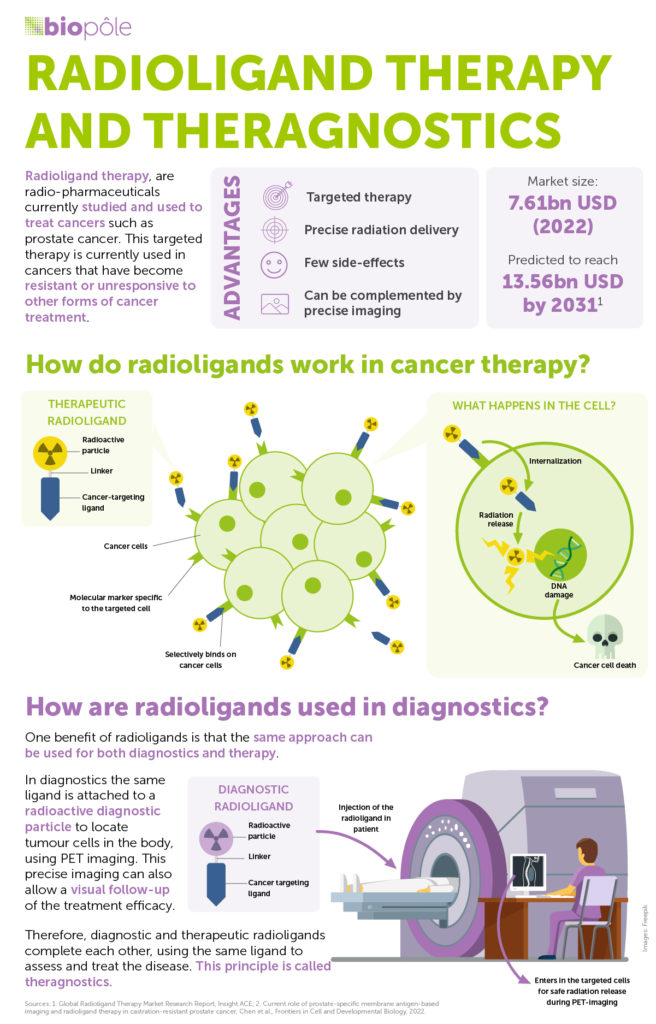
RLT, which harnesses nuclear power to treat cancer, is truly remarkable. By delivering radiation to very specific areas of the body, it destroys cancerous cells while aiming to limit the damage to surrounding tissue. This is made possible by using ‘ligands’, molecular structures that specifically bind to cancer cells, and ‘radionuclides’, that deliver radiation to damage targeted cells.
RLT holds immense medical potential. At Novartis, we expect it to become a major pillar of oncological treatments in the coming years. But to unlock this potential, we’ll need to overcome some initial challenges that are inherent to treating patients with radioactive therapy. Whether in hospitals, healthcare systems or manufacturing companies, the processes involved in RLT are vastly different to those involved in traditional cancer therapy. This is due to complex logistics, the need for specialised skills and infrastructure and the additional layer of regulations that governs radioactive substances.
A race against time
First, let’s look at the logistics of delivering this radioactive treatment to patients. RLT is made at manufacturing sites, like those run by Novartis in Italy and Spain. Given its radioactive nature, RLT has a short shelf life: it loses strength over time. This is a key consideration. It means the strength of each dose will depend on the distance from the manufacturing site to the treatment centre. It also means precise timings are crucial. Unlike other medicines, we can’t stock RLT treatment and only have a few days to get it from the manufacturing site to the treatment centre.
So, once the treatment is manufactured, it must be transported right away. Again, the radioactive nature of RLT makes this a delicate operation, requiring specialised transport and specific customs clearances.
The complex logistics of RLT
Once the treatment reaches a hospital, it must be administered to the patient in a shielded room. And in Switzerland, the patient cannot be discharged until 48 hours after receiving the therapy. Even though the radiation involved in RLT travels only tiny distances – mere millimetres – these precautionary measures are taken to protect healthcare workers and caregivers from the radiation that is present in a patient’s body following treatment. Radiation levels decrease rapidly, so it is considered safe to discharge patients after two days.
In addition, we need dedicated infrastructure and resources to deliver RLT treatment. Hospitals need to plan and construct rooms where patients can shield. They need the right imaging equipment and the right disposal system for radioactive waste. And above all, they need the right people. From oncologists to nuclear medicine physicians, radiologists, radio pharmacists and nurses, a whole team of specialists must be in place and working in perfect coordination for a patient to be treated. It’s what we call ‘healthcare system readiness’.