Cell therapy has the potential to cure virtually any disease, including cancer and HIV. But right now, it’s very expensive and there isn’t enough manufacturing capacity to reach all the patients who could benefit from the treatment. Luc tells us more about co-founding Limula – based at Biopôle’s StartLab – to help solve the manufacturing problem in this sector.
Where did the idea for Limula come from?
It all started in 2018 when my Co-Founder, Yann Pierson, met a clinician working on CAR-T cell therapy at the University of Geneva. This professor spoke about the difficulty of producing sufficient cells for future clinical trials. Yann realised the bottleneck was hugely limiting in this sector and there was a considerable appetite for innovations that could lead to scalable production and more patients getting access to a cure.
In true ‘garage start-up’ fashion, Yann spent months at home working on a first concept and prototype before filing a patent. In the late summer of 2019, he showed me a video of his invention and asked whether I thought I could help him unlock its potential. I’m a chemist by training but had been involved in synthetic biology and immuno-oncology research for a few years, and suddenly I had found a real-world application for these methods that could have a huge impact on patients. So I quit my job and have never looked back.
How does your tool help patients who undergo cell-based therapy?
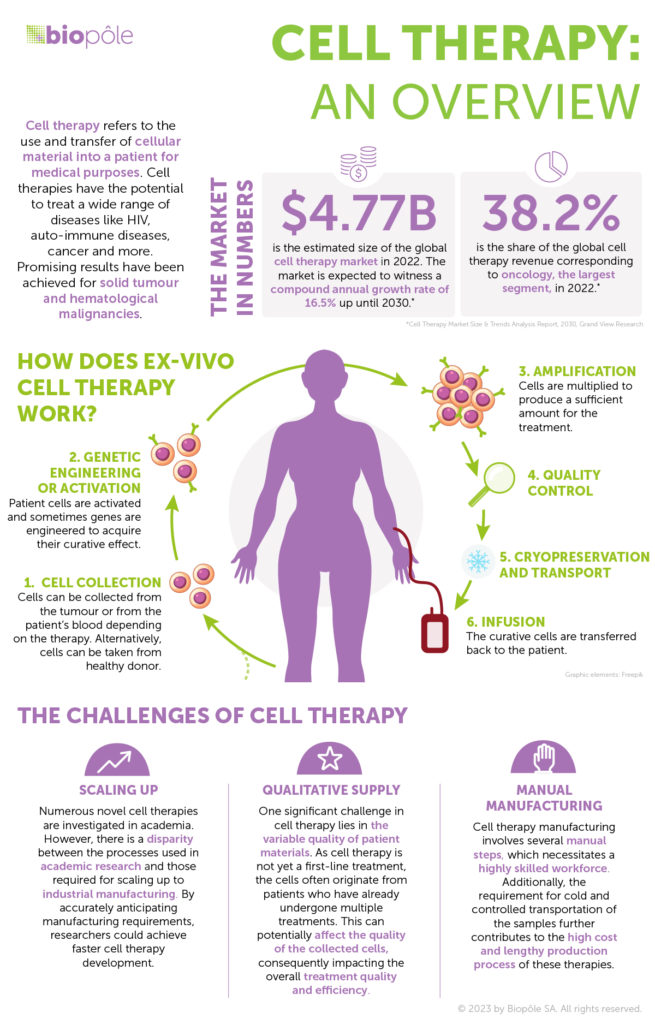
The production of cell therapies is currently very manual: stem cells or immune cells are collected from the bloodstream, frozen and shipped to a pharma manufacturing facility. The engineered cells are manufactured there using multiple devices, frozen again and shipped back to the hospital where the patient is waiting. It’s a very complex and expensive process and there just isn’t the capacity to bring this treatment to all the patients who need it.
We believe widespread access will only happen when we have a scalable and standard process. Limula provides a tool that automates multiple steps to increase manufacturing capacity. We deliver process intensification by removing some manual steps and decreasing the amount of equipment needed for a given process.